Active ingredient: Pyrethrins
Other name: 'Evergreen' (MGK); 'ExciteR' (Prentiss); 'Milon' (Frunol); 'Pyrocide' (MGK); 'PY-T-20' (Botanical Resources); 'PY-T-50' (Botanical Resources)
Formulation: Pyrethrins 1.5% EW
IUPAC name: for pyrethrin I, Roth: (Z)-(S)-2-methyl-4-oxo-3-(penta-2,4-dienyl)cyclopent-2-enyl (1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate; or
(Z)-(S)-2-methyl-4-oxo-3-(penta-2,4-dienyl)cyclopent-2-enyl (+)-trans-chrysanthemate;
for cinerin I, Roth: (Z)-(S)-3-(but-2-enyl)-2-methyl-4-oxocyclopent-2-enyl (1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate; or
(Z)-(S)-3-(but-2-enyl)-2-methyl-4-oxocyclopent-2-enyl (+)-trans-chrysanthemate;
for jasmolin I, Roth: (Z)-(S)-2-methyl-4-oxo-3-(pent-2-enyl)cyclopent-2-enyl (1R)-trans-2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropanecarboxylate; or
(Z)-(S)-2-methyl-4-oxo-3-(pent-2-enyl)cyclopent-2-enyl (+)-trans-chrysanthemate
CAS No.: [121-21-1] pyrethrin I; [25402-06-6] cinerin I; [4466-14-2] jasmolin I
Molecular weight: pyrethrin I: 328.4; cinerin I: 316.4; jasmolin I: 330.5
Molecular formula: pyrethrin I: C21H28O3; cinerin I: C20H28O3; jasmolin I: C21H30O3
Structural formula: 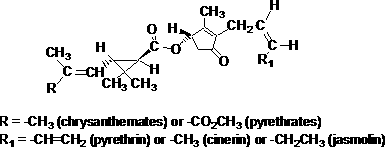
Mode of action: Non-systemic insecticide with contact action. Causes rapid knockdown (caused mainly by Pyrethrins II), with death occurring later (associated with Pyrethrins I). Has some acaricidal activity.
Uses: Control of a wide range of insects and mites in public health, stored products, animal houses, and on domestic and farm animals. Control of chewing and sucking insects and spider mites on fruit, vegetables, field crops, ornamentals, glasshouse crops, and house plants. Also used as personal insect repellents and for human head lice control. Normally combined with synergists, e.g. piperonyl butoxide, which slows down detoxification by the insects.